- Meeting requirements and proving competency through numerous supplements and rejections... New challenging opportunities in Malaysia, India, Romania, etc.
[Medical Newspaper/Daily Newspaper = Reporter Oh In-gyu] Due to a series of accidents over the years, the European Union has strengthened its management and supervision of certification in the medical device field and presented strict requirements and review standards, and cases are having difficulty getting over the barrier. It has been increasing recently. However, there is a domestic company that has overcome this challenge and obtained CE certification after several challenges, and has proven its quality, performance, durability, and safety.
Based on this, it is expected that in the future, there will be more exports led by AdipoLABs to many other countries since it is relatively easier to obtain certification in other countries than Europe.
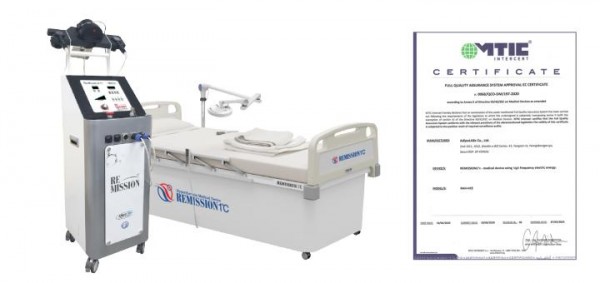
AdipoLABs (CEO Han Sung-ho) announced on the 12th that the company has obtained medical device European CE certification for its flagship product, the medical high-frequency hyperthermia device of ‘REMISSION 1℃’.
Previously, starting in the second half of 2018, AdipoLABs conducted certification through MTIC, located in Italy. MTIC is an international certification body that certifies a wide range of systems, products and personnel to meet the requirements of international standards, European directives (CE marking) and national regulations.
In the first quarter of 2019, AdipoLABs collected CE data in English and applied for a CE application by submitting electromagnetic compatibility test samples, biological test samples, and RoHS samples, starting with a meeting with a domestic consulting company (KMC).
There were difficulties in receiving certification due to several supplements and rejections over the course of a year, but after review by MTIC Italy in March, the final copy of CE certification was delivered on the 8th of this month.
AdipoLABs' high-frequency hyperthermia cancer treatment device, REMISSION 1°C, received manufacturing approval from the Ministry of Food and Drug Safety in 2015, Halal certification in 2018, US FDA registration, ISO13485 certification, Shariah certification, and RoHS certification. This is a definite recognition of technological capabilities.
REMISSION 1℃ is a medical device that not only improves blood circulation and immunity by raising the body temperature above 40℃, but also treat cancer and relieve pain. Since January 2019, the equipment has been introduced to Seoul St. Mary's Hospital and has been receiving attention from the medical community as it has shown many positive clinical effects.
The response at major exhibitions in Asia was also enthusiastic. The device was exhibited at the 'Middle East and Asia Business Expo 2018' held in October 2018 and the '35th International Medical Equipment and Hospital Equipment Exhibition (KIMES 2019)' held in March 2019, attracting the attention of domestic and foreign medical officials.
In addition, REMISSION 1°C received extra attention at a special seminar in Xi'an, Shaanxi Province, China in September of the same year and at the ‘2019 World Halal Day’ in Samara, Russia in October.
Sales begin at Malaysia's largest hospital, expected to take a leap forward on the global stage
Meanwhile, for the company that has been struggling due to the impact of COVID-19, obtaining the European CE serves as a stepping stone for a leap forward onto the global stage.
AdipoLABs, which has been focusing on overseas sales with partners in each country, has received CE certification, acquiring an opportunity to speed up sales activities in Southeast Asia, India, Mauritius, Romania, etc. for export contracts.
In particular, in the case of Malaysia, with the CE certification of REMISSION 1℃, it is possible to immediately obtain permission for REMISSION 1℃ as a medical device from the Ministry of Health (MDA) in the country, and sales will be made to each hospital. Currently, the REMISSION 1℃ has been undergoing clinical trials on cancer patients at UMMC (University Hospital Malaysia) since June and is showing positive results. Based on these results, local approval as a cancer treatment medical device is expected.
UMMC is a Malaysian semi-governmental hospital and the largest hospital in the country. Professor Ho Gwo Fuang, who is in charge of clinical practice, is a professor who is academically recognized not only in Malaysia but also around the world.
In addition, a company official pointed out that Beacon Hospital, the largest private cancer center in Malaysia, is expected to purchase a large amount of REMISSION 1℃ in a short period of time as AdipoLABs has obtained CE certification.
CEO Han Sung-ho said, “Despite the difficult conditions at home and abroad due to the COVID-19, we received CE certification through the efforts of our executives and staff. In the future, exports of medical high-frequency hyperthermia device of REMISSION 1°C will gain momentum,” adding, “We are currently experiencing a slump in overseas sales and domestic sales. I hope that the second half of this year will be a year of growth for the company, putting the first half of the year behind us.”
Reporter Oh In-gyu 529@bosa.co.kr
Source: http://m.bosa.co.kr/news/articleView.html?idxno=2132275