
Local officials are looking at AdipoLABs' 'REMISSION 1℃' at the event of 'Introduction and demonstration of innovative cancer treatment equipment in Korea' held at Oxymed Hospital in India.
The high-frequency hyperthermia cancer treatment device, “REMISSION 1℃,” developed with pure domestic technology is emerging as a new hope for the treatment of cancer patients. In particular, India's 1.3 billion pair of eyes are set on this newly introduced REMISSION 1℃ lately.
AdipoLABs' (CEO Han Sung-ho) high-frequency hyperthermia cancer treatment device, ``REMISSION 1℃'' that was developed in order to free the cancer patients from extreme pain of disease was introduced on the Indian newspaper, The Hindu and then on the media including Thanthi TV. Soon, curiosity arose and REMISSION 1℃ intrigued the entire nation of India.
"The Hindu" is an Indian daily newspaper with an average of 1.2 million copies sold, and "Thanthi TV" is a news channel broadcast in Tamil Nadu, India.
The media discussed in depth about the launching and trial event of South Korean Innovation Cancer Treatment Equipment. The event was held on November 15th at Oxymed Hospitals in Chennai, India.
Organized by AdipoLABs and sponsored by the Oxymed Foundation, Oxymed Hospitals' Social Responsibility Division, the United World Halal Development (UNWHD) and the KOTRA's Social Responsibility Project, the event became successful with Sun-gi Kim, KOTRA Chennai Trade Center Director, AYAZ AKBER, Hospital Director of Oxymed Hospitals, MOHAMED JINNA of United World Halal Development (UNWHD), T. RAFEEQUE AHMED in Chennai TAW, and TAMILSELVAM COO of United Health Tourism, all attending with lots of others from government and hospitals.
In particular, AYAZ AKBER, Director of Oxymed Hospitals, drew a lot of attention from attendees by presenting an improved case of treating patients with 'REMISSION 1℃'.
In addition, the daily newspaper, The Hindu said Oxymed Hospital is now working with AdipoLABs in Korea to challenge cancer. This system uses high-frequency hyperthermia to raise body temperature and safely enhance cellular function without stimulating sensory and motor nerves.”
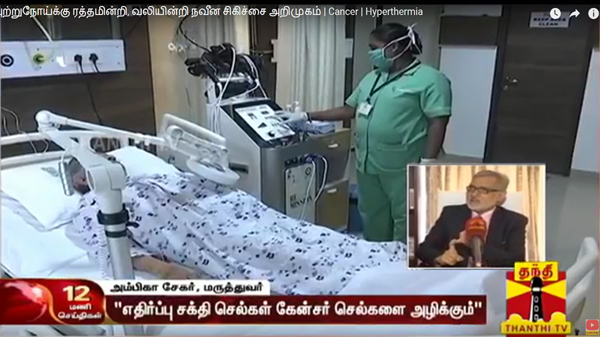
▲ 'Thanthi TV', a news channel aired in Tamil Nadu, India, introduces AdipoLABs' 'REMISSION 1℃'.
In addition, in 'Thanthi TV', AYAZ AKBER, Director of Oxymed Hospital, who actually uses 'REMISSION 1℃', explains the treatment method, the process, and the result of using 'REMISSION 1℃'. The interview with the patient's guardian also received tremendous attention. He said “It has been only 2 weeks since the procedure was performed with REMISSION 1℃, but the patient already feels much better and aware his cough has decreased.”
'REMISSION 1℃' is a medical device manufactured and produced by AdipoLABs based on pure domestic technology. In October 2015, it was licensed as a high frequency hyperthermia medical device to treat cancer by the Ministry of Food and Drug Safety in South Korea.
'REMISSION 1℃' obtained Halal certification on May 1st, registered with the US FDA on August 1st, and acquired ISO13485 certification on October 23rd for entering Europe.
'REMISSION 1℃' has a mechanism of necrosis of cancer cells that are weak to heat by generating deep heat through high frequency electricity in the human body. It is becoming a new hope of treatment for those suffering cancer patients in Korea and other countries.
AdipoLABs also visited the Office of Sirdariyah in Uzbekistan and the National Cancer Center of Gulystan at the capital of Sirdariyah from November 15th-17th along with Dr. Il-bong Choi who is the president of Korean Society for Thermal Medicine. Local personnel and medical staff were well educated about hyperthermia heat treatment at their visit and there was also an interview about "REMISSION 1℃" at a government owned media, UZBEKISTAN 24.
“We are pleased to spread and transfer Korea's latest hyperthermia treatment technology to India and Uzbekistan at this time. I strongly hope there won't be any more suffering cancer patients in the world due to hyperthermia treatment of REMISSION 1℃. I hope that more countries start using REMISSION 1℃ very soon." said Han sung-ho, CEO of AdipoLABs.
As such, AdipoLABs' 'REMISSION 1℃', a high-frequency hyperthermia medical device developed with purely domestic technology, is currently being used for the treatment of patients in 50 locations, ranging from small and medium-sized hospitals upto a member of big five hospitals in South Korea. Following this, interest in the Indian market is expected to increase, leading to a significant increase in overseas exports.
